
An old time combination A & B radio battery
*Batteries*
Walk into any retail establishment and you'll be confronted by a wide array of batteries. There is little information to assist you in determining which battery is best for your needs. I'm going to take a crack at clearing out the fog.
Batteries come in sizes, voltages and types. There are rechargeable batteries, disposable batteries and disposable batteries that can be recharged. First we'll look at the sizes of the more common batteries. Generally we have
For this article I'm not going to discuss the button cells, gel cells or lead acid batteries. You may still be amazed at just how complicated this will get.
All the batteries listed with letter sizes (AA, C, D, etc) come in either 1.5 volts for disposables or 1.2 volts for rechargeables. Generally speaking the two can be used interchangeably. Products intended to run on batteries are designed to function across a range of voltages. What is most important is the ability to deliver current over time and rechargeables have a great advantage here.
Trivia alert!
Yes Virginia, there were once A cells and B cells. You can still get A cells in Canada and B cells in Europe. They are big heavy batteries. A cells were meant to heat the filament of a vacuum tube and ranged from 1.5 to 9 volts, while B cells ranged from 22.5 to 90 volts and supplied constant positive voltage to the plate. (I once had a naval surplus low level radiation meter that required the 22.5 volt battery.) A portable tube device would also use C cells for grid bias voltage. Only the C cell survived, mainly for intermediate sized flashlights and later for intermediate powered boom boxes.

An old time combination A & B radio battery
There are also F cells that are slightly less than twice the length of 2 D cells and a host of fractional sizes like 1/3 AA or 3/2 D. Consumers almost never see these.
A "cell" is the basic device for the production of electricity. Although originally a battery referred to several cells ganged together for higher voltage or greater current capacity, today a single cell is also called a battery. Most people, including battery manufacturers, today treat the two terms as interchangeable.

From left to right D...C...AA...AAA...9 volt
The most commonly used general purpose batteries are identified by a letter designation: N, AAAA, AAA, AA, C and D,
N cells look like a half length AA cell and give 1.5 volts. There is a similar sized 12 volt battery (Duracell calls it an MN21) that is used in alarm remotes and cigarette lighters. It is a stack of 18 tiny coin sized cells and is often mistaken for an N.
AAAA cells are the smallest of the cylindrical batteries. They are as long as an AAA but slimmer. Six of them are ganged together in a 9 volt battery. Some of the smaller consumer personal electronics are starting to use them.
AAA and AA cells are the most commonly used sizes today. Modern consumer electronics demands a combination of smaller size than the larger D and C and higher current than the 9 volt battery can supply. Higher voltage isn't as necessary to drive modern electronics. They are beginning to control more of the flashlight market as extremely bright LED flashlights become more common.
C cells and D cells are slowly falling out of favor. Their primary use is in larger flashlights and portable "boom boxes".
There is also a "sub-C cell" most commonly found ganged together in rechargeable tool batteries and shavers. You won't find these in most retail shops, they are used by manufacturers.
And as mentioned above there are 9 volt rectangular, which are really 6 batteries ganged together in series and 6 and 12 volt lantern batteries which are sub-Cs ganged together in different combinations. They have been around a very long time. The lantern batteries have experienced a resurgence due to their use in fluorescent lanterns. The 9 volt batteries are mainly used in smoke detectors, some radios, some radio control equipment and assorted other gadgets. They are also great fun to touch to your tongue.
Other batteries such as tool batteries are typically sub-C cells ganged together in series as this illustration shows:

Rechargeable power tool battery
I put a rechargeable AA next to it for a size comparison. If your rechargeable tool battery dies on you, you may not be able to find the replacement part. That happened to me when my Coleman Powermate power tool set batteries died and I learned they'd changed the battery configuration. (Power tool manufacturers work hard to make each tool/battery combination unique in its fit so that nobody comes up with a generic battery replacement.) The new configuration did not fit in the old tools and I was unable to find it anywhere. My solution was to find the closest battery I could find in shape to the one I needed and perform a battery transplant. It took a bit of work to reconfigure the cells to the exact shape I needed but now my tools are no longer useless.
It was cheaper to buy a flashlight that was a part of a power tool system with the battery than to buy the battery by itself. Go figure. You'll notice that all tool batteries are in multiples of 1.2 volts, the voltage for a standard size rechargeable battery. Another alternative would have been to buy rechargeable sub-C cells with solder tabs on each end and build the pack from scratch. That way I could have used the longer lasting NiMH instead of old style NiCads. You can fix a lot of rechargeable stuff when it dies this way. Shavers and such often don't have consumer friendly replaceable batteries so when the battery croaks for good, it's either fix it yourself, send it to a repair shop if you can find one or replace the entire unit (what the manufacturer really wants).
You won't find sub-Cs and solder tabbed batteries and odd sized at most retail outlets. You have to hunt for these at specialty electronics like Electronics City or on line at places like All-battery.com. I'll talk later about what to look for in a rechargeable.
One of my FRS radios uses 3 rechargeable N cells ganged in series. Here's a AA and an N cell for a size comparison.

3 N cell battery pack
Another uses 4 AAA disposable batteries instead. I could just substitute rechargeables for the disposables with no loss in performance AND longer battery life. Even though the voltage is lower, it's the current capacity that counts.
My cordless telephone uses 4 AAA rechargeables in series. If I needed to I could kludge together my own pack of disposable or rechargeable batteries to replace them. Shown is an AAA in comparison.

4 AAA battery pack
3 volt lithium cells were the first lithium batteries on the consumer market They are referred to as a CR123 size (a little smaller in size than a C cell) and pack 3 volts. Some cameras and other electronic gadgetry use them. One example is a night vision scope I bought several years ago.
If you find you need a battery and you don't have the correct size, any other battery of the similar voltage can be used instead. There are a number of folks who make shells to convert AAA into AA and AA into D and C cells. Green Batteries is one. The most common use is for people who only have an AA sized charger and still want to power their D and C cell devices. They'll work just fine as long as you understand they won't last as long. If all you had were a handful of AAA cells you could indeed power your 7 D cell Maglite using these adaptors. It would only last about 1/10 as long, but it would initially still shine as bright.
When looking at batteries there are a few terms you need to know. All units are derived from the names of famous electrical scientists of the past.
Assuming all batteries are identical for simplicity, when batteries are line up nose to tail - positive terminal to negative terminal - and the voltage is taken from the top positive terminal and rear negative terminal they are said to be in series. In this case the final voltage is the sum of all the voltages of the individual batteries but the current capacity is only equal to one battery. If they are beside each other and all the positive ends are joined together and negative ends are joined together they are said to be in parallel. In this case the current capacities are added together while the voltage is equal to that of one battery.


Where P is the power dissipated in Watts, E is the applied voltage in Volts, I is the current produced in Amps and R is the load resistance in Ohms:
Let's say that the voltage is 1.5 Volts and the circuit is 10 Ohms. The equation E=IxR can be rearranged to show I=E/R. How much current is flowing? I=1.5/10, so I is .15 amp in this case. How much power is it dissipating? P=IxIxR so P=.15x.15x10 = .225 Watt.
Zinc Carbon is "old school". They are dirt cheap and have been around since the dawn of the 20th century. They still work perfectly well in low current items like Walkman, MP3 players, flashlights, TV remote controls or small transistor radios,and the like, just won't last as long as the newer batteries. They may be the most economical battery for such an application if you don't mind the frequent changes. Inexpensiveness is balanced out by a short shelf life (1 1/2 years) and slightly higher rates of leakage. When I was a kid I remember a device we used to recharge 9V carbon-zinc radio batteries. It worked but battery life after recharge was fairly short.
Zinc Chloride is an updated Zinc Carbon. This are those "super heavy" duty batteries using slightly different chemistry and purer ingredients.
Alkaline - Manganese Dioxide is the most common battery in use today. It is much longer lasting in every application than either of the first two mentioned. Still at higher loads, like a digital camera, they will die very quickly. At a constant 1 amp drain they may last only 40 minutes while at a 1/10 amp drain they might last 30 hours. (Because of this wide variability, they don't print a current capacity on the label of any disposable battery.) All alkalines have a sloping discharge rate meaning that you see a gradual loss of power over time until the battery drops below a useful level. (Duracell says this is about .8 Volt.) As a point of fact, Duracell, Rayovac and Eveready standard alkaline batteries perform identically while off brands may perform weaker. I suspect this is due to poor quality control and cutting manufacturing corners. Here are curves comparing Rayovac's AA heavy duty zinc chloride battery performance against their standard alkaline. You can see that alkalines last more than twice as long as heavy duty. Ten ohms is a typical small CD player load.
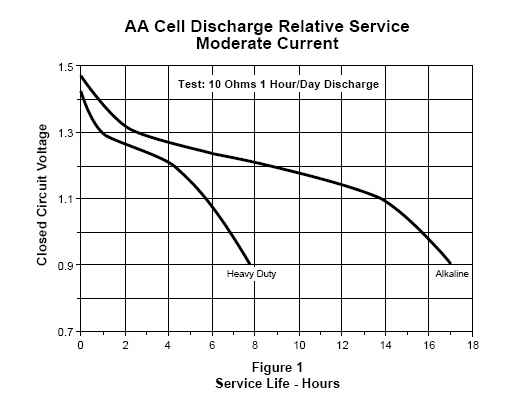
Here's another chart giving typical AA alkaline lifetimes with typical loads. (The flashlight uses a PR-2 bulb.) See how the amount of current capacity varies wildly depending on the load. Note also how much less current capacity there is when powering a digital camera.
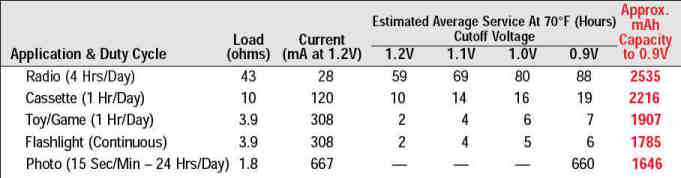
And here's duty life curve for Duracell CopperTop alkalines. This curve is in terms of load current instead of load resistance but it you remember E-IxR you can figure out what the load resistances must be:
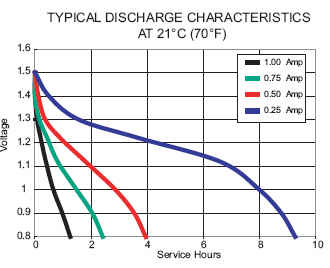
Duracell Coppertop alkaline
There is a gadget called the "BatteryXtender" claiming to recharge regular alkaline batteries but I have no solid information on it. Wikipedia says that regular alkalines are more prone to leakage when recharged this way but I have no FNV on this.
There are also alkalines out that are designed to be rechargeable in special chargers. They have the advantage of holding a charge for many years but do not have the high current capacity of their nonrechargeable brethren. They would be best for long term storage.
"Premium" Alkaline - Manganese Dioxide batteries are much hyped. Examples are Eveready's "e2 Titanium" and Duracell's "Ultra Digital". The manufacturers have added something proprietary (possibly titanium) that reduces the internal resistance slightly allowing for a little more current. When used in a digital camera my experience is that they still die quickly. These curves are for the Duracell "Ultra" AA alkaline battery:
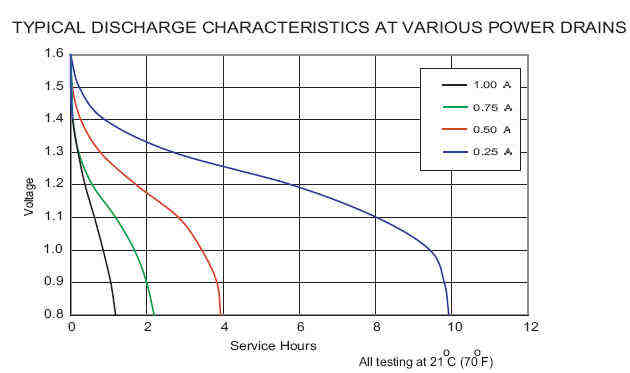
Duracell "Ultra" alkaline
Compare with the same chart above for a standard alkaline. Find out the price difference. Calculate if the increase in current capacity is worth the extra money.
Nickel Oxyhydroxide is the newest kid on the block. Examples are Duracell's PowerPix and Panasonic's Oxyride. Provides significantly more current than alkalines. They were designed for people with digital cameras in mind who didn't want to spend the bucks for lithium. I was unable to find any good data on them.
Lithium - Iron Disulphide - These are the most common consumer lithium batteries. They come in all the standard sizes. They last vastly longer than any current disposable in high current applications but have no advantage over alkaline in low current applications. They also have a big advantage in shelf life - 10 years and hold their charge longer and so are good for devices you tend to forget about like smoke detectors.
Lithium ion cells are like those used to power cell phones and laptop computers and always come in custom configurations. They have very high energy density, meaning the most amp-hours packed into a given weight. They also have a tendency to overheat or even catch on fire if they are shocked or used in such a way they cannot get adequate cooling.
Nickel Cadmium (NiCad) are the older style of rechargeable batteries declining in use because the cadmium is considered toxic waste when the battery finally fails. They also typically have half the current capacity of NiMH batteries of the same size. However for a few uses their ability to deliver a LOT of amps VERY quickly are useful. They are more rugged than NiMH, take more recharges and will last longer on the shelf, important for survival planning. Disadvantage is the development of "memory" through the formation of internal crystals which prevents the battery from accepting a full charge if not periodically discharged completely.
Nickel Metal Hydride (NiMH) is the new kid on the block and beating all rechargeable comers in energy density and current capacity, except for lithium ion. It has a flat discharge rate meaning power stays at a given level and then drops quickly as the battery is exhausted. They do not develop memory problems as NiCads do.
There really aren't many "deals" on batteries out there. I spent some time examining prices vrs. current capacity and the two seem to be proportional. Remembering that disposable batteries by brand name manufacturers are pretty much the same, Rayovac is much the best in terms of bang per buck in my neck of the woods. Store specials and rebate offers may change this calculation.
The current capacity of a battery is measured in milli-Amp-hours or mAh. A thousand mAh battery will supply 1000 mA (or 1 Amp) for one hour before it goes dead - theoretically. In reality different types of batteries have different discharge curves. And the manufacturers DO NOT make it easy for the average consumer to compare.
Notice that if you drain the power at a 1 Ampt rate, you get a little over 1 hour before the thing becomes useless. (Duracell has decided this happens at .8 V but will vary by application.) That's a little over 1 Amp-hr. of capacity. But if you discharge at a 1/4 Amp rate you get almost 10 hours of useful service life. That's almost 2.5 times more capacity.
Important fact: Batteries will produce more total energy running a low demand device for a long time than a high demand device for a short time.
Another related fact: Due to their chemistry, batteries have the ability to self-recharge somewhat after every usage if allowed to rest. (Short spurts of high current usage with breaks in between average out to the same thing as a low current over the same total time.) The fewer rest periods, the less you'll recharge action you'll get. If you run them down to nothing, you lose that self recharge ability almost completely.
Important fact:Batteries of all types are best stored between 32°F to 50°F. Higher and lower storage temperatures will shorten battery life. They should always be allowed to come to room temperature before use as the chemical reactions take place best in that general range. Very high (over 150°F) temps can cause them to explode. If you wish to store batteries over several years you should tightly (not vacuum) seal them in something like aluminized mylar. Batteries breath and exposure to low humidity for long periods of time will dry the contents out. On the other hand extended exposure to high humidity can cause rust and/or leakage.
Important fact: All batteries can leak. They are not sealed hermetically, they need to breath and can even dry out in very low humidity. Manufacturing shortcuts, high humidity, high heat, shock and old age are the primary factors. I once bought a large quantity of "Super Dear" batteries (they looked similar to Duracell coppertops) thinking that the manufacturing of alkaline batteries was standardized enough that an off brand would be okay. They did NOT last as long as regular Alkalines and had a VERY high leakage rate. It is important NOT to leave batteries in any unused device over time or the device may be damaged/destroyed if the battery leaks.
Rechargeable batteries come in two flavors, Nickel Metal Hydride (NiMH) and Nickel Cadmium (NiCad). There is no reason ever to use NiCads any more except price. NiMH has a higher current capacity per unit volume, accepts more recharge cycles before dying and are classified as toxic waste. The Cadmium in NiCads is poisonous, much more so than lead. The primary use of NiCads today is in battery packs and often have no informational labels at all. Many have solder tabs. Here is a picture of a NiCad C cell and a sub-C cell:

Unmarked Nicad C-cell

Rechargeable sub-C with solder tabs

Which one has more current capacity?
You'll notice that we have 2 batteries, both the same type (NiMH) same size (AA) and if you read the label, different capacities, 2500 mAh for the Eveready vrs. 2000 mAh for the Radio Shack. What gives? The truth is that not all batteries are created equal. There is no standardization in construction as there is with zinc and alkaline batteries. Manufacturers have to put the current capacity on the labels of consumer batteries. Since they are rechargeable, manufacturers count on your not reading the label AND not noticing the shorter time between recharges. Depending on the manufacturer, AA batteries can range in capacity from 1800 to 2900 mAh. (I find the lower the capacity, the smaller the type face they use.)
It gets even worse when dealing with larger sizes. Take a look at this chart:
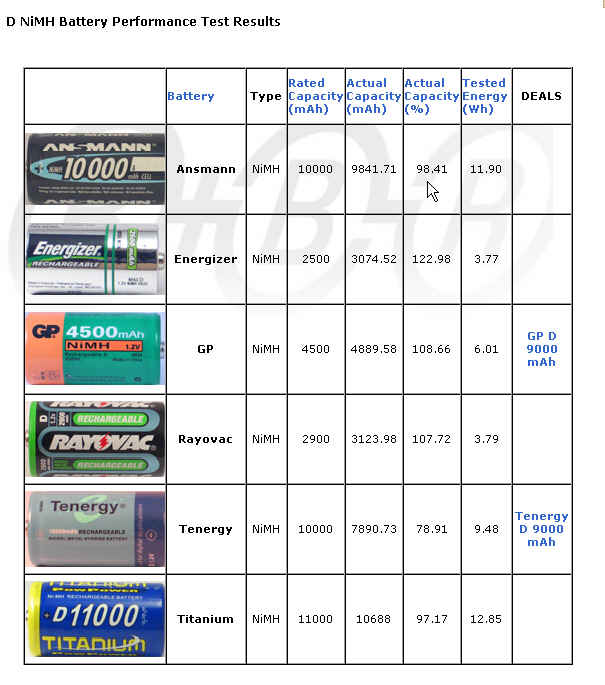
Different capacities from different manufacturers
The range is from 2500 mAh to 11,000 mAh. How can this be? Most consumer oriented rechargeable batteries larger than AA are just AA or sub-C cells packaged in a larger container. Do they charge the same price? Heavens no! The manufacturers count on your not doing comparison shopping. They have a rationale for this. Often people never really run down their rechargeable batteries, but rather they self discharge over time. This is the curse of rechargeables, a very limited shelf life between charges. After 28 days they may have lost anywhere from 10 to 70% of their fully charged capacity depending on the quality of manufacture.
A second problem is that some manufacturers have better quality control than others. Consequently some won't deliver the capacity listed (78-122% of rated capacity in the figure above). You can try testing yourself or rely on third party testing. The figure above is from rechargeable-battery-review.com.
The great blessing of NiMH batteries isn't as much the total amperage available but rather the peak current in Amps possible. A common alkaline battery of the same size may well have more total amp-hours available, but as we've seen will die very quickly under a high current demand. There is no advantage to NiMH in moderate to low current draw devices. Batteries have internal resistance which adds to the resistance of the load.
As long as Rbattery is small compared to Rload it is usually ignored. Typical internal resistances are .15 Ohm for NiCad, .32 Ohm for Lithium Ion (that's why they are preferred for many electronic applications) .75 Ohm for NiMH. When you put a very low resistance load across your battery the internal resistance will prevent the current from going beyond a certain level. NiCad batteries have very, very low internal resistance and are still preferred by many for electronic flash applications where short bursts of very high current are needed to charge large capacitors quickly.
Should you buy the highest capacity battery? If they are kept charged and then used in high demand emergency devices for extended periods without recharge, certainly - if you can afford them. If they are used for recreational purposes, well maybe not. You're best economy will come from dividing the cost of a battery by it's current capacity to come up with a cost per amp-hour. An 10,000 mAh battery is probably not a bargain if it costs 10 times as much as a 2,500 mAh battery.
When you buy a rechargeable battery, it should be fully charged and discharged a couple of times before being put to general use. Rechargeables slowly gain efficiency over time and may not reach their peak capacity until have been cycled hundreds of times. However only five cycles are needed to bring one up to written specs.
Do not leave your NiMH or NiCad batteries in the charger. (This runs counter to how they are actually used in the real world. When not in use, most rechargeable devices live on their chargers.) The charger keeps them hot and that is not good for them. It better to leave them out and cycle them though the charger every couple weeks. NiCad chargers can cause NIMH batteries to explode. NiMH chargers are safe for NiCads however. NiCads can be trickle charged while NiMHs like a fast charge. Also keep them out of the sun and hot cars.
Exercise caution if attempting to recharge standard alkaline or Zinc-carbon batteries. Overcharge is VERY easy and can lead to leakage or explosion.
This is just my humble opinion but...
Fred
www.alpharubicon.com
All materials at this site not otherwise credited are Copyright © 1996 - 2007 Trip Williams. All rights reserved. May be reproduced for personal use only. Use of any material contained herein is subject to stated terms or written permission.