Alpha and Beta radiation are common types of radiation. The alphas and the betas have distinct properties, one of the most important is that they are little particles which are created with a starting velocity, and they slow down. Since they are continuously slowing down, eventually they will stop, and will have a finite range.
Alphas Particles
Alpha particles are the emitted by heavy isotopes and consist of two protons and two neutrons. Often an alpha particle is referred to as a Helium nucleus. The typical energy of an alpha particle is around 4 to 8 MeV. A MeV (pronounced Em ee Vee) is an abbreviation for a million electron volt of energy, which is not a terribly intuitive unit for most people. The MeV is a unit of energy, but an every day analogy might be to think of the weight of a car or a truck 1 ton is on the light side, and so is 1 MeV of particle energy. 10 tons of truck is respectable, so is 20 tons. By comparison 20MeV is a far whack of energy for most particles. Particle energies can go a lot higher, but you won’t find them around outside a laboratory or in outer space. This might sound like a fairly weird comparison, but when I was little, we always talked of “doing a ton” meaning travelling100 mph. If that’s still too weird, think of the radiation and ignore the energy: “that’s a 6 or a 7 alpha particle”.
Betas Particles
Beta particles are high energy electrons produced form radioactive isotopes. Most beta energies are less than 1 MeV, but there are a few higher energy beta particle emitters.
Both alpha particles and beta particles have an electric charge. This means that an alpha particle and a beta particle are continually influenced by the electrons of the material that they are traveling through. Because they are charged an alpha particle and a beta particle will continuously loose energy and slow down in any material. Because of that, beta particles and alpha particles have a finite distinct range.
Range in Air
The range in air of alphas and betas is estimated by various formulas.
Figure 1 presents the range in air of alpha particles and Figure 2 presents the range in air of beta particles. Different sources have been used, although they agree pretty well with each other. Alpha particles are much more massive than electrons, and so when they move through the air they tend to ionize everything. Because of this the alphas might start out with a lot more energy than a typical beta, but they will slow down and stop a lot faster. Since most typical alpha particles are less than 10MeV, a rule of thumb would be a hand span away will remove the danger of most alpha particles. Beta particles will travel longer distances, up to a few meters so 20 feet is going to be the maximum range of any betas that we will encounter in practice. We should keep in mind that after such a long distance the betas will have lost a lot of energy, so their penetration in tissue will have fallen off quite a bit.
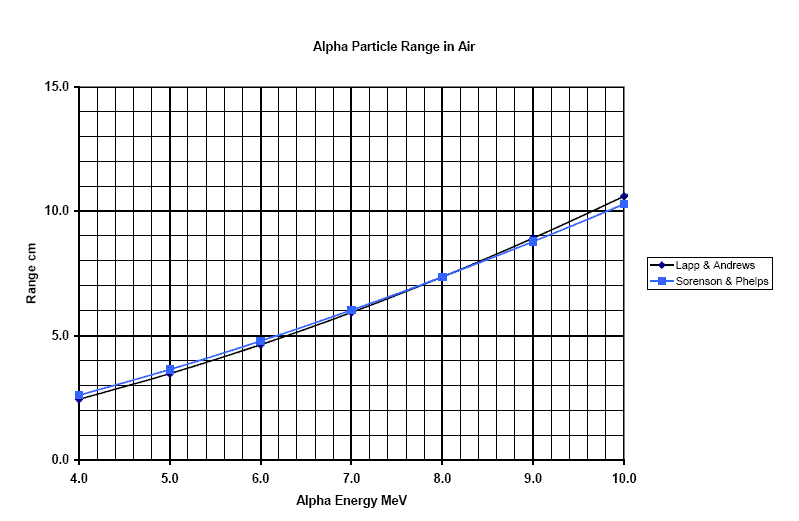
Figure 1
Range in air of Alpha particles of various
energies. The distance is in units of centimeters, and the energies are in
terms of MeV.
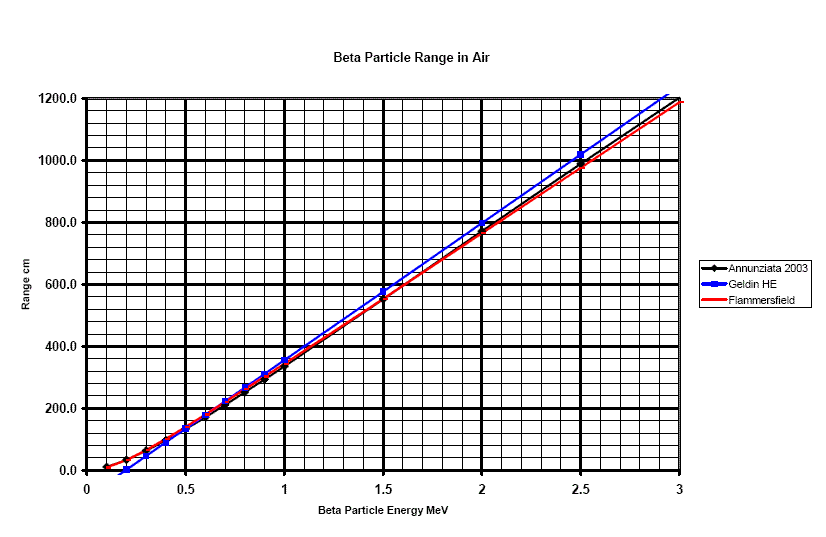
Figure 2
Range in air of beta particles. The distance is in
units of centimeters, and the energies are in terms of MeV. Note how much
longer beta particles will travel.
Examples
The maximum energy of beta particles form the isotope Phosphorous 32 is 1.710 MeV. What is its range in air?
Ans: From figure 2, the range in air of a beta particle of that energy is about 650 centimeters (roughly 20 feet).
Americium 241 (used in smoke detectors) emits alpha particles of energy 5.5 MeV. What is their range?
Ans: From Figure 1, the range of an alpha particle of 5.5MeV energy is about 4 cm in air.
Range in water
Water is a lot denser than air, and is a pretty common material. Most plastics are about the same density as water, and most solids are equal or denser than water. Humans are pretty close to water in average density too. So the range in water can be considered as a maximum worst case for shielding.
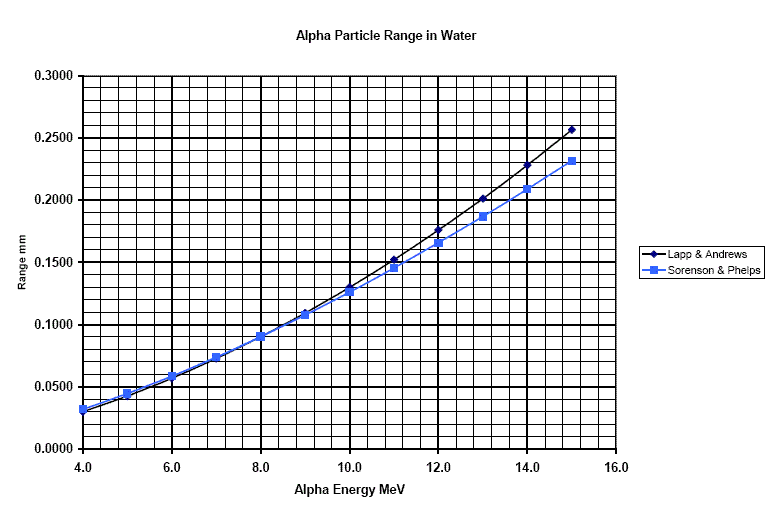
Figure 3
Range of alpha particles in water.
This displays the miniscule range of alpha particles in
water. Note that the range is in millimeters.
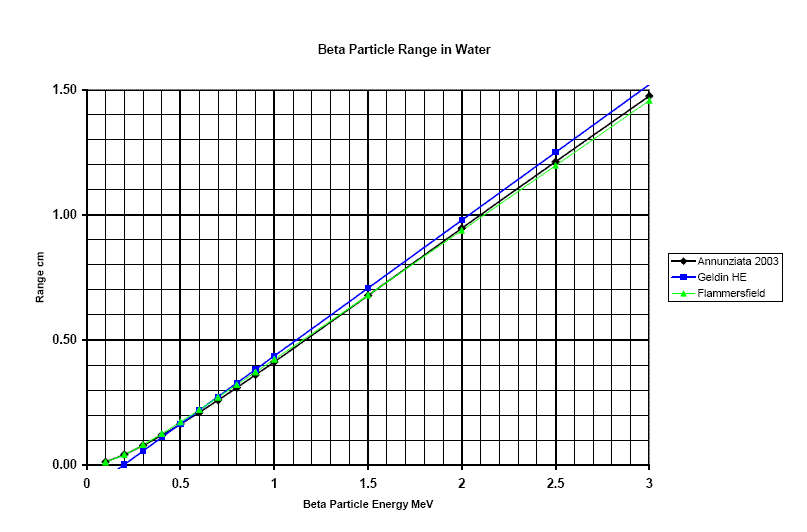
Figure 4
Range of beta particles in water.
Examples
Polonium 210 is sprayed all over the place from a radiological weapon. The energy of the alpha particles from Polonium 210 is 5.3 MeV. What range will these alpha particles have in water or skin tissue? Light cotton clothing about 1 mm thick is being worn by a person. Will alpha particles penetrate the cotton clothing?
Ans: from figure 3, the range of an alpha of energy 5.3 MeV is less than 0.05 millimeters in water. Even an alpha particle of three times the stated energy only penetrates a quarter of a mm. The skin surface will not be penetrated by the alphas, and neither will 1 mm of cloth. They can however be left on the surface as contaminant dust and become ingested.
The TSA decide to invest in area beta monitors distributed by the Radiological71 security company. These are craftily marketed as having a range of 10 meters. What energy beta would you need to be able to detect it from 10 meters in air?
Ans: A range of 10 meters is 1,000 centimeters. From figure 4, the corresponding energy is about 2.5MeV.
A person carrying a plastic bag of Strontium 90 walks past one of the Radiological71 security companies beta detectors. The average energy of Strontium 90 is 0.54MeV. How close do they have to be to be detected?
Ans: From figure 4, the an energy of 0.54MeV for betas corresponds to about 150 cm (1.5 meters or bout 5feet)
Example Alpha Energies
| Isotope | Half Life | Units | Alpha Energy (MeV) |
|---|---|---|---|
| 210 Bi | 5.013 | days | 4.687, 4.650 |
| 206 Po | 8.8 | days | 5.2237 |
| 208 Po | 2.898 | years | 5.1149 |
| 210 Po | 138.4 | days | 5.30433 |
| 222 Rn | 3.8235 | days | 5.48948 |
| 241 Am | 432.2 | years | 5.5445, 5.48556, 5.44280 |
Example Beta Energies
| Isotope | Half Life | Units | Alpha Energy (MeV) |
|---|---|---|---|
| 32 P | 14.3 | days | 1.710 |
| 3 H | 12.3 | years | 0.0186 |
| 90 Sr | 28.8 | years | 0.546 |
Contaminating x-rays and complications…
Most of the time 241Am decays via alpha emission. However, it does occasionally decay by giving off a low energy beta particle (0.052MeV range in air about 5 cm) or occasionally a gamma ray. This means that although you cannot measure any alpha particles (or beta particles) a foot or so away from a 241Am source, there may be some contaminant x-rays.
Also, in the process of slowing down, electrons can give off a small amount of x-ray radiation. This is called slowing down or braking radiation, but is often referred to in the German for braking radiation as Bremsstrahlung radiation.
The braking radiation isn’t very significant in low atomic number materials (water, air, plastics, people, ex-wives etc). This is because a low atomic number nucleus doesn’t have many protons, so the overall charge is small, and the force to brake the electron is relatively low. A heavy nucleus contains a large amount of charge, and so has much more braking force on a passing electron.
A rough analogy for the braking radiation is to imagine an electron as a car braking, and think of the noise as the x-rays given off. Low atomic mass nuclei are like very small speed bumps, so if you started the car off on a speed way at say 100 mph and put it in neutral, you would have to go over a lot of speed bumps to slow the car. Each time the car passes over the speed bump there is a little bit of a thud. On the other hand an electron and a really heavy nucleus is like a car hitting a concrete barrier. If the car runs into that, there is a very rapid deceleration and a lot of noise. When it hits a heavy nucleus (like a lead or tungsten atom) an electron emits a lot of x-ray radiation.
Certain isotopes are pure emitters, for example Strontium 90) and its decay products, which are themselves radioactive) is almost a pure beta emitter. Others, like Radium 226 decays via alpha decay, but also gives of a large amount of high energy gamma rays. Another example is Iodine 131, which is a common product in reactor disasters, fallout from weapons (tests or otherwise). Iodine 131 emits via beta decay, and also emits gamma radiation. In the case where gammas are emitted, there is still a good chance of something being detected at a long range. For example, a roadside radiation meter will not pick up any betas from a passing car containing Iodine 131, but it will likely detect the gammas that are emitted by the Iodine.
Summary
Alphas have the shortest range or any radiation, and won’t get further than a few inches in air.
Betas can travel up to a few yards in air, or a fraction of an inch in water.
If a source is a pure alpha or pure beta emitter, then the radiation will be stopped by quite thin shielding.
References and further reading
Formulas
E is the energy of the radiation in MeV units, and the resulting range is the range in cm.
The range for electrons can be scaled by the density of the material they travel through [Ann 2003]
Lapp and Andrews formula for Alpha range in air: (0.005 E + 0.285)E3/2
Sorrensen and Phelps formula for Alpha range in air: 0.325R3/2
Annunziata electron range in water: 0.412 E (1.265 -0.0954 ln E)
Gelndein HE formula of alphas in air: 0.542E - 0.133
Flammersfield 1946 formula electrons in water: 0.11[Sqrt(1+ 22.4E2)-1]
Online:
[Ann 2003] Handbook of Radioactivity Analysis 2nd EditionMichael F L'Annunziata 2003
[Shapiro 2002] Radiation Protection Harvard University Press Fourth Edition 2002 Jacob Shapiro
[EPA 2009] The Environmental protection agencies web-page on alpha particles.
[EPB 2009] The Environmental protection agencies web-page on beta particles.
[lbl 2009] Lawrence Berkely National Laboratory listing of the values of: A. Rytz, At. Data and Nucl. Data Tables 47, 205 (1991).
Radiological71
www.alpharubicon.com
All materials at this site not otherwise credited are Copyright © 1996 - 2009 Trip Williams. All rights reserved. May be reproduced for personal use only. Use of any material contained herein is subject to stated terms or written permission.