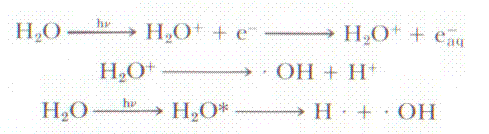*Radiation Protectors*
By: Radiological71
6 November 2008
1 Introduction
2 Oxygen enhancement ratio
3 Dose reduction factor
4 Amifostine
5 So what’s the problem? (And Summary)
Introduction
Some substances will confer an amount of protection from
radiation.
One of the first radiation protectors discovered was cysteine.
When given to mice it was found doses about 1.8 times higher than that of a
control group were needed to give the same level of mortality. One of the
problems with cyseine is its toxicity, as it produces nausea and vomiting if
taken if the amounts required to provide any protection from radiation.
Consequently, the US army started synthesizing large numbers of compounds at
the Walter Reed army hospital (about 3,000 were tried) and one of the drugs
developed was Amifostine. Amifostine is the inactive form of the compound, (activation
is by dephosphorylation to the metabolite WR 1065 by the plasma membrane bound
enzyme alkaline phosphatase). How does a chemical provide any radiation
protection? And are they worth the trouble? To understand the effects we need
to take a slight detour into the effect of oxygen on cells.
Oxygen Enhancement Ratio (OER)
Cells that contain different amounts of oxygen have
different sensitivities to radiation. For human cells, the deficiency of oxygen
protects the cells from radiation, and increasing the oxygen increases the
cells sensitivity to radiation. Because of its effect, oxygen is referred to as
a radio-sensitizer. A simple experiment can be carried out to illustrate the
sensitivity of cells. This is to irradiate two groups of cells, one under
normal conditions, and other under conditions of increased oxygen. The ratio of
the dose required to kill the cells with the oxygen divided by the amount to
kill cells without oxygen is referred to as the oxygen enhancement ratio (OER).
OER = (dose need to kill cells without oxygen) / (dose to
kill cells without oxygen).
For example, suppose 50% of a group of cells were killed
with 300 RAD and the dose needed to kill identical cells with some reduced
level of oxygen was 600 RAD. The oxygen enhancement ratio would be 600/300 = 2.
This does sound a bit backward, since removing the oxygen “enhances” the dose
that can be applied, but if we think of the enhancement in the killing power of
the radiation it makes sense. Add oxygen, enhance the killing power, hence an
OER. Figure 1 shows a graph taken from [Hall 1994]. The maximum OER for human cells
in a Petri dish is about 3, meaning the maximum difference in radiation doss
for the same effect between full oxygen and zero oxygen conditions is a factor
of 3.
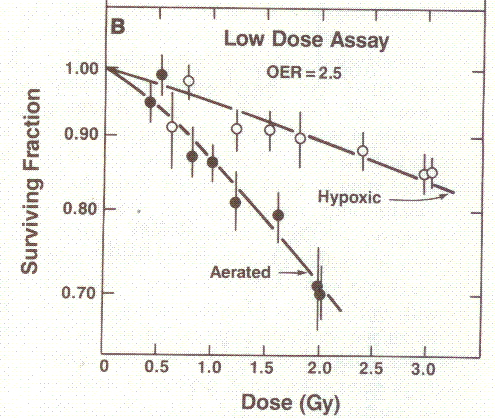
Figure 1: An illustration of the oxygen enhancement ratio for
x-rays. Adding oxygen (denoted as aerated) to the cells makes them more
sensitive to the radiation, or enhances the radiation’s killing power, hence we
talk of an oxygen enhancement ratio. Shamelessly reproduced from [Hall 1994].
Dose Reduction Factor
From the above, if oxygen is removed from cells, the effect
of radiation is reduced. The OER can be understood, at least a little bit, by
understanding what happens when radiation interacts with material.
When x-rays or gamma rays interact in the body, they produce
fast moving electrons which then create various reactive species, such as free
radicals. From [Johns 1983] a few of the chemical reactions are shown. Once the
free radicals are produced, they immediately begin interacting with the
surrounding chemicals, one of which is DNA. Now DNA can be affected by direct
photon ionization, or by the electrons produced by the ionizing radiation, but
most of the interaction are from the free radicals.
Scavenging the free radicals with some other chemical,
"mopping them up" before they can do more damage will reduce the effect of the
radiation. Effectively, this is how the radiation protectors discussed here
work. Because some of the electrons and gamma rays will hit the DNA anyway, there
will be some damage, no matter how efficient the radiation protector was.
Additionally, gamma rays are sparsely ionizing radiation. A gamma ray has a lot
of penetration because it gives up its energy in relatively small amounts.
Neutrons or other radiation, because they act like giant bowling balls smashing
everything in there path tend to overload any scavenging of free radicals.
Consequently, we would not expect a radiation protector to
have more effect than removing the oxygen altogether. The maximum protection
for a perfect scavenger would be about three.
The dose reduction factor (DRF) is the dose for a given lethality with the drug divided
by the dose for the same effect in the absence of the drug.
DRF = (dose for a given effect with protection) / (dose for the same effect)
for example if 600 RAD produced 50% cell kill with a certain drug, and 300 RAD produced the same cell death without the drug the DRF would be 2.
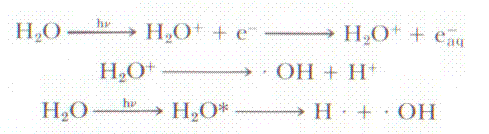
Figure 2: Some Free radical reactions caused by ionizing
radiation. Aqueous electrons very rapidly bind to oxygen and form various free
radicals and ions. Examples are from [Johns 1983]
Amifostine
Several different radiation protector drugs exist, here I
will write mainly of amifostine since amifostine has a wide current medical
use. Amifostine is a quite common radiation protector drug. Several years ago Amifostien
was regularly used most lung cancer treatments during radiation therapy.
Amifostine scavenges free radicals produced by radiation and
inactivates active species through formation of thioether conjugates. The
scavenging of reactive oxygen species (ROS) has been noticed in TBI treatments
[Facorro 2004]
Amifostine has been approved by the USA FDA as a radioprotector
[FDA 1999]. Amifostine is also known as "Ethyol". Lunar astronauts were given
the chemical for an emergency use.
Amifostine has been used to protect normal cell function
during chemotherapy [Lurruso 2003] and during combined chemo-radiation therapy
[Movsas 2005]
Amifostine is reported as being usually well tolerated, with
the most clinically significant side effect (dose-limiting toxicity) being
hypotension [Devita 1997]. Others would add that fevers, chills and sweating
are common effects too. The subcutaneous method rather than IV tends to be more
tolerated, for example [Kouk 2003].
As an example of clinical use, in China [Liu 1992] two
groups of patients with rectal cancer were treated with radiation, one with and
one without amifostine. With amifostine no rectal toxicities were seen, with
out it, 14% of the patients had some rectal and bladder toxicity. Other results
include reduction of pain in swallowing following treatment for lung cancer [Sarna
2008].
What is of interest here is not the medical uses for
localized areas, but the effects of these radiation protectors for whole body
irradiation. Total body irradiation is used for different therapies, such as
leukemia and bone marrow transplant [Facorro 2004], Gabriel [2000]
The results of amifostine are detectable in large groups.
In mice, the factor of protection from total body irradiation approaches three.
The main clinical justification is that tumor vascularization is not as
complete as normal tissue. This means that blood will carry the amifostine to
the normal tissue, and that the amifostine will only diffuse slowly into the
tumor. If amifostine is given a few minutes before some radiation therapy, the
normal tissue will be protected, and the tumor will not be affected. Also, amifostine
affords sparing to the gastrointestinal lining and the salivary glands quite
well due to the hydrophilic nature of the drug, but doesn’t cross the blood-brain barrier
to protect the brain.
So what’s the problem? (And Summary)
Well, radiation protection can be shown for mice, and for
medical therapy. Since amifostine acts by scavenging the free radicals, it must
be given before the radiation. It is of no use in radiation protection after
the event. Amifostine and sulfhydryl groups will be broken down in the stomach,
so a tablet isn’t going to help. This means a subcutaneous injection (usually
the stomach) or an IV. The Soviet army use to carry Cystaphos, a similar sulfhydryl
chemical to amifostine, but it was provided in tablet form. Useful? No. Unlike KI,
which can be given to a general population as a tablet, a radiation protector will
require a measured dose delivered by IV or subcutaneous injection.
Summary Points:
Decreasing the Oxygen concentration makes a cell more
resistant to radiation
Radiation protectors can scavenge free radicals produced by
radiation and protect cells
The maximum radiation protection would be a factor of three.
Radiation protectors have to be given before a radiation exposure
References and further reading
Offline
[Devita 1997] Devita et al, Cancer, principles and practice
of oncology, page 3089, 5th edition Lippencott-Raven.
[Hall 1994] Hall, E. Radiobiology for the Radiologist 4th edition Lippencott
and Williams 1994 ISBN 0-397-51248-1
[Johns 1983] Johns and Cunningham The Physics of Radiology 4th
edition. Charles C. Thomas. ISBN 0-398-04669-7
[liu 1992] Liu T, Liu Y, He S et al.Use of radiation with or
without amifostine in advanced rectal cancer. Cancer 1992; 69: 2820-2825
Online
[Hall 2006] Hall, E. Radiobiology for the Radiologist, 2006.
http://books.google.com/books?id=6HhjwRyqBzgC&pg=PA131&lpg=PA131&dq=amifostine+lunar+missions&source=bl&ots=0sfByGffJ2&sig=lV-qLkzNZEAFUegsz2sQtQf97Eo&hl=en&sa=X&oi=book_result&resnum=1&ct=result
[Lorusso 2003] D. Lorusso et al, Phase
III multicenter randomized trial of amifostineas cytoprotectant in first-line
chemotherapy in ovarian cancer patients, Annals of Oncology 14:1086-1093,
2003
http://annonc.oxfordjournals.org/cgi/content/full/14/7/1086
[Movsas 2005] B. Movsas et al, Randomized trial of amifostine
in locally advanced non-small-cell lung cancer patients receiving chemotherapy
and hyperfractionated radiation: radiation therapy oncology group trial 98-01
J Clin Oncol 2005 April 1;23(10):2145-54.
http://www.ncbi.nlm.nih.gov/pubmed/15800308?dopt=Abstract
[Sarna 2008] L Sarna et al, Clinically Meaningful
Differences in Patient-Reported Outcomes With Amifostine in Combination With Chemoradiation
for Locally Advanced Non-Small-Cell Lung Cancer: An Analysis of RTOG 9801.
Int J Radiat Oncol Biol Phys.2008 May 21.
http://www.ncbi.nlm.nih.gov/pubmed/18501528?dopt=Abstract
[Facorro 2004] G. Facorro et al, Oxidative study of
patients with total body irradiation: effects of amifostine treatment
Bone marrow transplantation 2004,vol.33,no8,pp.793-798
http://cat.inist.fr/?aModele=afficheN&cpsidt=15625225
[Gabriel 2000] D. Gabriel et al, Use of Amifostine to Reduce
Mucositis Following Total Body Irradiation (Tbi)-Based Autotransplants for
Lymphoma Proc Am Soc Clin Oncol 19: 2000 (abstr 268A)
http://pediatricca.asco.org/portal/site/ASCO/menuitem.34d60f5624ba07fd506fe310ee37a01d/?vgnextoid=76f8201eb61a7010VgnVCM100000ed730ad1RCRD&vmview=abst_detail_view&confID=2&index=y&abstractID=201368
[FDA 1999] FDA Amifostine datasheet
http://www.fda.gov/Cder/foi/label/1999/20221s12lbl.pdf
[Koukourakis 2003] Koukourakis M I, Amifostine before Chemotherapy
Improved Tolerance Profile of the Subcutaneous Over the Intravenous Route Clinical
Cancer Research Vol. 9, pages 3288-3293, August 2003
http://clincancerres.aacrjournals.org/cgi/content/full/9/9/3288
Radiological71
www.alpharubicon.com
All materials at this site not otherwise credited are Copyright © 1996 - 2009 Trip Williams. All rights reserved. May be reproduced for personal use only. Use of any material contained herein is subject to stated terms or written permission.